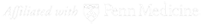Projects / Techniques
Understanding the mechanisms of novel genetic epilepsy syndromes of childhood. Investigators in the Goldberg Lab recently discovered de novo mutations in the sodium channel gene SCN3A as a novel cause of severe epilepsy of infancy (epileptic encephalopathy) and are using advanced neuronal models to understand the mechanisms of SCN3A encephalopathy and attempt to develop novel “precision” approaches to pediatric epilepsy.
See Zaman, T., Helbig, I., Božović, I.B., DeBrosse, S.D., Bergqvist, A.C., Wallis, K., Medne, L., Maver, A., Peterlin, B., Helbig, K.L., Zhang, X., Goldberg, E.M.: Mutations in SCN3A cause early infantile epileptic encephalopathy. Annals of Neurology 83(4):703-717, 2018. VIEW PDF
Cellular architecture of epileptic cortical networks. The lab utilizes two-photon calcium imaging to probe the structure and function of cortical networks in epilepsy. At left, video (5-times speed) depicting activity in layer 2/3 primary somatosensory cortex while an experimental subject runs on a treadmill, imaged using the genetically-encoded calcium indicator GCaMP6f (green; GENIE Project, Janelia Research Campus) under control of a synapsin promoter. A subset of GCaMP-expressing cells are presumptive parvalbumin-positive putative fast-spiking neocortical interneurons (red, labeled with the red fluorescent marker tdTomato.
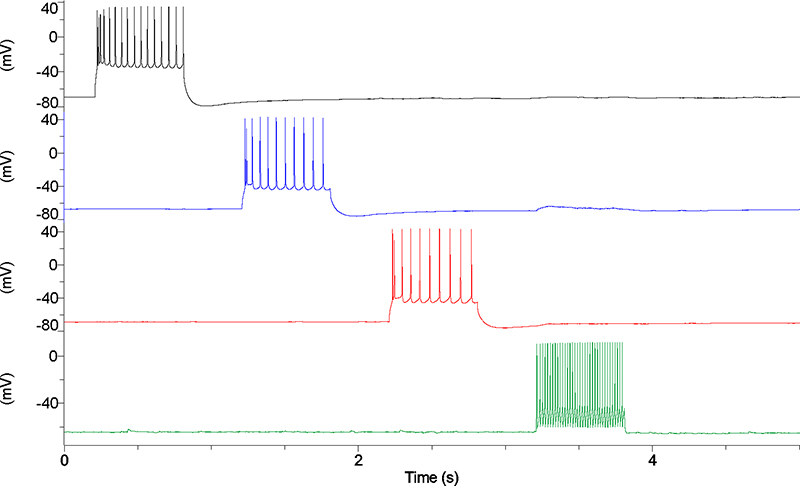
Circuit-level mechanisms of epilepsy. We use two photon calcium imaging, electrophysiology, and opto- and chemicogenetics, in acute brain slices, to study inhibition in cerebral cortical circuits as well as circuit dysfunction in experimental epilepsy models. At left is shown labeling of granule cells in the dentate gyrus with GCaMP6f (A; green), expression of tdTomato-tagged Archaerhodopsin T (ArchT; courtesy of E. Boyden, MIT) in parvalbumin-positive putative fast-spiking interneurons in dentate gyrus (B; red), and the superposition (C). The combination of large-scale dynamic imaging of cortical circuits with two-photon calcium imaging, combined with the ability to manipulate defined subsets of neurons, allows for detailed interrogation of cortical circuits in the normal and diseased brain.
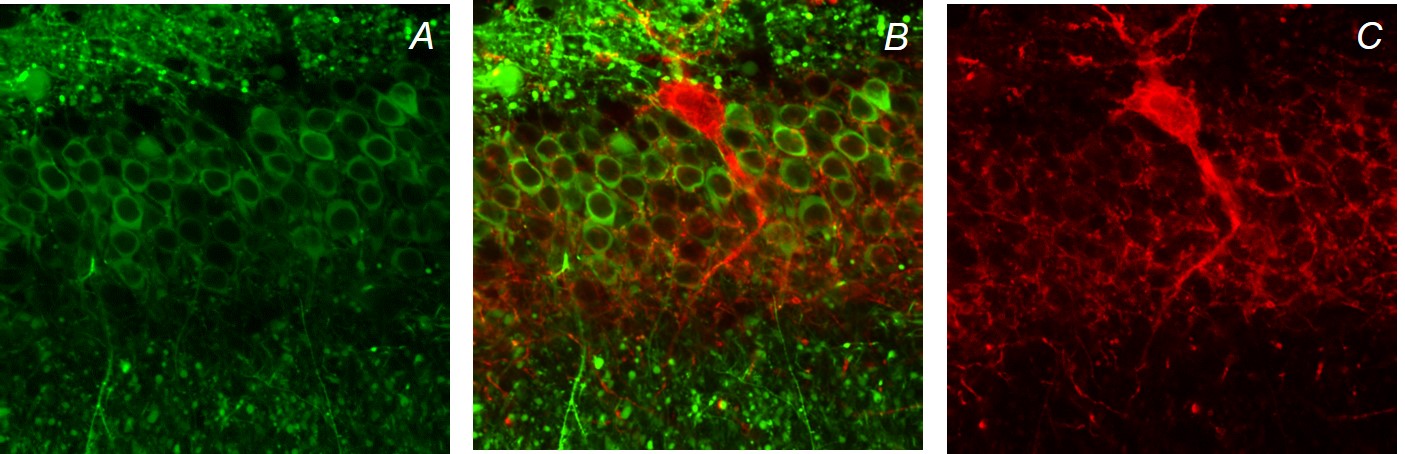
Treatment/prevention of epilepsy using stem cell-derived cerebral cortical interneuron progenitors. In collaboration with the Laboratory of Stewart A. Anderson, MD, also at CHOP/The University of Pennsylvania, we are attempting to treat and prevent epilepsy in experimental models of acquired focal epilepsy as well as severe infantile-onset genetic epilepsies using progenitors of specific subtypes of cerebral cortical interneurons derived from embryonic stem cells.
At left is early-stage stem cell-derived interneuron progenitors at 3 weeks post-injection (green) showing immunopositivity for the interneuron marker somatostatin (red), co-stained with DAPI (blue).